Wednesday, January 30, 2008
Hypothesis
These researchers believe that through Boyles' Law, which states at a constant Kelvin Temperature the pressure and volume of a gas are inversely proportional, that after fuel is poured into our cannon the temperature will cause the the pressure to build up and cause the volume to decrease and cause the ball to explode out. This will be similar to a syringe effect where as pressure builds the volume becomes smaller and forces the liquid out, except our cannon will be using temperature.
Monday, January 28, 2008
Construction
Planning
After our original plan was formed we began to sketch the cannon. 

Our original funnel sketch depicted the rough dimensions of the top chamber.
Our next sketch was a representation of the entire cannon.
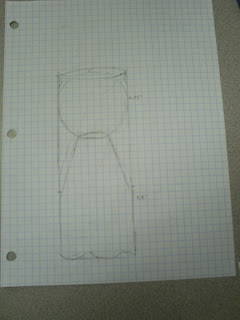
After realizing the dimensions of the launched object would be different, we decided to shorten the length of the top chamber to let the projectile rest on the top of the tube.


Our original funnel sketch depicted the rough dimensions of the top chamber.
Our next sketch was a representation of the entire cannon.

After realizing the dimensions of the launched object would be different, we decided to shorten the length of the top chamber to let the projectile rest on the top of the tube.
Research
Former Knowledge
According to Boyle's Gas Law, as volume decreases pressure increases. In accordance with this law, we sought to minimize the volume of our cannon while providing an area for the gas to build up. By making the bottom chamber small with a funnel at the top we planned for the gas to build up while making its way through the funnel. The funnel, blocked by the tennis ball, would trap and contain the gas, until eventually the ball would shoot off.
P1 x V1 = P2 x V2
As Pressure ^ Volume v
As Pressure v Volume ^
P1 x V1 = P2 x V2
As Pressure ^ Volume v
As Pressure v Volume ^
Subscribe to:
Comments (Atom)



